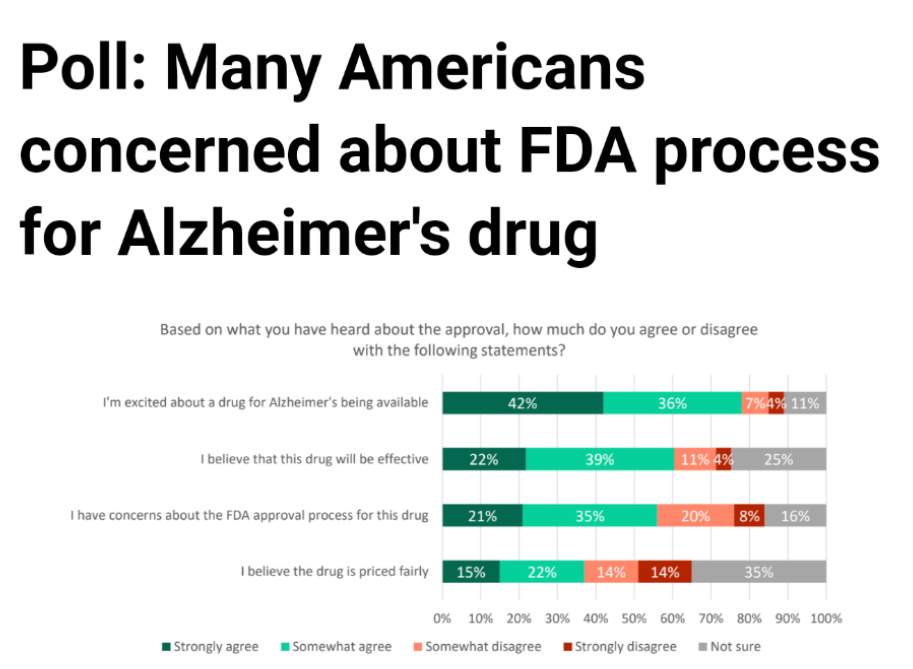
Survey by The Harris Poll, 6/11/21-6/13/21, via Statnews
As the American population continues to live longer by the year, more people are suffering illnesses resulting from age-related changes in the brain; Alzheimer’s disease is one of the most common of these age-associated illnesses. On June 7th, 2021, Biogen’s Aducanumab (Aduhelm) was approved by the FDA to treat all stages of Alzheimer’s, nearly eighteen years after the administration approved Namenda for the disease’s severe stages. According to The Wall Street Journal, of the 6 million Americans diagnosed with Alzheimer’s, over 1 million can be eligible to take Aduhelm. So, why are so many healthcare providers, patients, and families steering clear of the Aduhelm infusion?
In this HealthCetera podcast, Barbara Glickstein, MPH, MS, RN, discusses the complexities of the FDA’s decision to approve Aduhelm with health reporter Liz Seegert, the Association for Health Care Journalists topic leader on aging. This interview follows the publication of “Alzheimer’s Drug Approved Monday by FDA Raises Questions for Journalists”, a co-authored two-part series on the Association for Health Care Journalists’ blog, written by Seegert and Tara Haelle, the medical studies core topic leader.
This interview was recorded on June 14, 2021, as a part of HealthCetera in the Catskills on WIOX radio.
Podcast: Play in new window | Download